Impact of screening for preeclampsia in the first trimester of pregnancy
A large multicenter French study assessing the value of screening for preeclampsia in improving the health of mothers and their children
Why the RANSPre study?
Preeclampsia is a complication that may occur in the second half of pregnancy and is characterized by arterial hypertension associated with proteinuria (presence of abnormally high amounts of protein in the urine). It affects 2% of pregnant women, that is 15 000 patients each year in France. This condition can potentially be serious for mother and child.
Preeclampsia is related to dysfunction of the placenta. There is currently no treatment of preeclampsia once it occurs, and only delivery – allowing the removal of the placenta – is associated with a regression of the disease. Sometimes an early delivery is necessary, exposing the child to the complications of prematurity.
There is a treatment that prevents the occurrence of preeclampsia : aspirin at low doses between 100 and 160 mg once a day. This treatment is more effective when started early in pregnancy, before 16 weeks of gestation. It is recommended in France for pregnant women who have had preeclampsia in a previous pregnancy.
Other preeclampsia risk factors are known, but at present time we do not know how they constitute an indication for aspirin. Never the less, several English-speaking countries recommend the prescription of aspirin in women based on these risk factors, which greatly increases the percentage of pregnant women who receive this treatment (up to 30%).
A screening test, («Fetal Medicine Foundation triple test ») can be used to estimate the risk of preeclampsia from the first trimester of pregnancy. It includes maternal clinical parameters (age, number of previous pregnancies, medical history, geographical origin, smoking, modalities of conception, weight, blood pressure), ultrasound parameters (blood flow resistance of the uterine arteries) and biochemical parameters (dosage of Placental Growth Factor in the maternal blood).
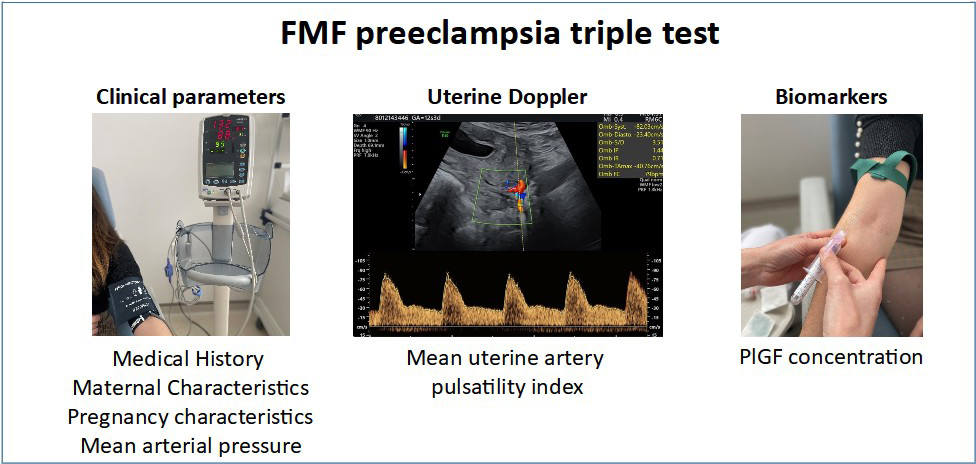
This combined screening test identifies women at risk of developing preeclampsia, in addition to those with a history of preeclampsia, and enables better targeting of the indications for treatment with aspirin during pregnancy. This screening is not currently included in routine care because the benefits and the potential side effects for mother and child are unknown. We do not yet have a randomized study (ie, with randomization) to assess this screening strategy and to compare women screened this way with women who receive the usual care.
What if early screening for preeclampsia reduced its incidence and complications?
What are the objectives of the RANSPre study?
The main objective of RANSPre is to assess the value of systematic screening for preeclampsia in the first trimester of pregnancy in terms of the health of mother and child.
The secondary objectives are to evaluate the impact of this screening on pregnant women’s satisfaction and on the costs of care.
RANSPre will also evaluate the predictive performance of the combined test in the French population as well as the feasibility of its implementation.
For more information: https://clinicaltrials.gov/ct2/show/NCT05521776/
Who can take part in the RANSPre study?
Any pregnant woman with:
- an ongoing singleton pregnancy between 11 and 14 weeks
- an age over 18 years
- social security coverage
Preeclampsia during a previous pregnancy and a contraindication to aspirin are non-inclusion criteria to the study.
What does the RANSPre study involve?
Participation in RANSPre is offered by the clinical team of the maternity hospital before the usual first-trimester ultrasound examination.
Each patient who agrees to participate in the study, a randomization is performed to determine in which group she will be cared for:
- Group A with preeclampsia screening
- Including a Doppler scan of the uterine arteries (on the day of or within three days of the ultrasound scan; click on image to see the explanatory video) and a blood sample

- Calculation of the risk of developing preeclampsia:
- Group A1 : If the screening test is positive (estimated risk of preeclampsia >1/100), aspirin treatment is started and continued until 36 weeks, and the pregnancy is followed according to the usual recommendations
- Group A2 : If the screening test is negative (estimated risk of preeclampsia <=1/100) the pregnancy is follwed according to the usual recommendations
- Including a Doppler scan of the uterine arteries (on the day of or within three days of the ultrasound scan; click on image to see the explanatory video) and a blood sample
- Groupe B without preeclampsia screening, with the usual management of pregnancy

RANSPre is a French study funded by the Ministry of Solidarity and Health (PHRC program). It is an academic study without industry funding.
The aim is to include 14 500 patients over 2 years, starting on 1 November 2022.
Participating maternity hospitals
21 maternity hospitals which are part of the Gynecology and Obstetrical Research Group (GROG) take part in the RANSPre study.
How to take part in the RANSPre study?
If you are interested in participating in RANSPre, your maternity hospital has to be part of the study. You can discuss this with your care team and/or contact us by email at: etude.ranspre.cch@aphp.fr
Here is the patient information sheet for the RANSPre study (french only): PATIENT INFORMATION SHEET
RANSPre team

Prof Vassilis TSATSARIS
Coordinating Investigator
Maternité Port Royal (Paris)

Dr Catherine DENEUX
Scientific Director
INSERM EPOPé Team (Paris)

Prof Jean GUIBOURDENCHE
Biologist director
Hôpital Cochin (Paris)

Natasha CARETTI
Coordinating midwife
Maternité Port Royal (Paris)
Sponsor: AP-HP
Partners of the RANSPre study








For more information on preeclampsia and the RANSPre study
The association Grossesse Santé contre la Pré-Eclampsie : https://www.grossesse-sante.org
A MOOC on preeclampsia: https://www.fhu-prema.org/enseignement/mooc-et-e-learning/mooc-pre-eclampsie/
INSERM preeclampsia dossier: https://www.inserm.fr/dossier/pre-eclampsie/
References
1. Chappell L, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. 2021 July 24;398:341-354.
2. Lecarpentier E, Fournier T, Guibourdenche J, Tsatsaris V. Pathophysiology of preeclampsia. Presse Med. 2016 Jul-Aug;45(7-8 Pt 1):631-7.
3. Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V. Aspirin for Prevention of Preeclampsia. Drugs. 2017 Nov;77(17):1819-1831.
4. Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol. 2020 Aug 21:S0002-9378(20)30873-5.
5. Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007 May 26;369(9575):1791-1798.
6. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018 Mar;218(3):287-293.e1.
7. Société française d’anesthésie et de réanimation (Sfar); Collège national des gynécologues et obstétriciens français (CNGOF); Société française de médecine périnatale (SFMP); Société française de néonatalogie (SFNN). Multidisciplinary management of severe pre-eclampsia (PE). Experts’ guidelines 2008. Société française d’anesthésie et de réanimation. Collège national des gynécologues et obstétriciens français. Société française de médecine périnatale. Société française de néonatalogie. Ann Fr Anesth Reanim. 2009 Mar;28(3):275-81.
8. Mounier-Vehier C, Amar J, Boivin JM, Denolle T, Fauvel JP, Plu-Bureau G, Tsatsaris V, Blacher J. Hypertension and pregnancy. Expert consensus statement from the French Society of Hypertension, an affiliate of the French Society of Cardiology. Presse Med. 2016 Jul-Aug;45(7-8 Pt 1):682-99.
9. Lecarpentier É, Vieillefosse S, Haddad B, Fournier T, Leguy MC, Guibourdenche J, Tsatsaris V. Placental growth factor (PlGF) and sFlt-1 during pregnancy: physiology, assay and interest in preeclampsia. Ann Biol Clin (Paris). 2016 Jun1;74(3):259-67.
10. Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009 May;53(5):812-8.
11. Wright D, Tan MY, O’Gorman N, Poon LC, Syngelaki A, Wright A, Nicolaides KH. Predictive performance of the competing risk model in screening for preeclampsia. Am J Obstet Gynecol. 2019 Feb;220(2):199.e1-199.e13.

